Graphite, dabarar kwayoyin halitta: C, nauyin kwayoyin: 12.01, wani nau'i ne na sinadari carbon, kowane carbon atom yana haɗe da wasu ƙwayoyin carbon guda uku (wanda aka tsara a hexagons na zuma) don samar da kwayoyin halitta.Domin kowane carbon atom yana fitar da electron, waɗanda ke iya motsawa cikin yardar kaina, don haka graphite shine madugu.
Graphite yana daya daga cikin ma'adanai mafi laushi, kuma amfaninsa sun haɗa da yin fensir da man shafawa.Carbon wani nau'in ƙarfe ne wanda ba na ƙarfe ba wanda ke cikin zagaye na biyu na ƙungiyar IVA na tebur na lokaci-lokaci.Ana yin graphite a babban yanayin zafi.
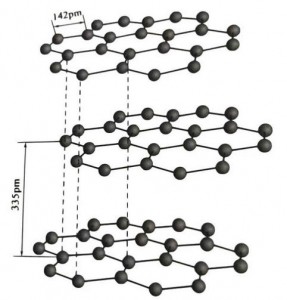
Graphite ma'adinai ne na kristal na abubuwan carbon, kuma lattice ɗin sa na crystalline tsari ne mai kauri mai kauri.Nisa tsakanin kowane layin raga shine 3.35A, kuma tazarar atom ɗin carbon a cikin layin raga ɗaya shine 1.42A.Tsarin kristal hexagonal ne tare da cikakkiyar tsage-tsalle.Fuskar da ke tarwatsewa ita ce haɗin gwiwar kwayoyin halitta, ba ta da kyau ga kwayoyin halitta, don haka yawo na halitta yana da kyau sosai.
A cikin lu'ulu'u na graphite, ƙwayoyin carbon a cikin wannan Layer suna samar da haɗin gwiwa tare da sp2 hybridization, kuma kowane carbon atom yana da alaƙa da wasu ƙwayoyin cuta guda uku a cikin haɗin haɗin gwiwa guda uku.Atom ɗin carbon guda shida suna samar da zobe mai ci gaba shida a cikin jirgin guda ɗaya, yana faɗaɗa cikin tsarin lamella, inda tsayin haɗin haɗin CC ɗin ya kasance 142pm, wanda yake daidai da tsayin tsayin ɗabi'ar atomic crystal, don haka Layer ɗaya. , shi ne wani atomic crystal.Carbon atom a cikin jirgin sama guda suna da p orbit guda ɗaya, wanda ke mamaye juna.Electrons suna da 'yanci, daidai da electrons kyauta a cikin karafa, don haka graphite zai iya gudanar da zafi da wutar lantarki, wanda shine halayyar lu'ulu'u na karfe.Don haka kuma an rarraba su azaman lu'ulu'u na ƙarfe.
An raba tsakiyar Layer na kristal graphite da 335pm, kuma nisa yana da girma.An haɗa shi da ƙarfin van der Waals, wato, Layer na da crystal na kwayoyin halitta.Duk da haka, saboda daurin carbon atom a cikin jirgin sama daya yana da ƙarfi sosai kuma yana da matukar wahala a lalata shi, wurin rushewar graphite shima yana da girma sosai kuma halayensa suna da ƙarfi.
Dangane da yanayin haɗin kai na musamman, ba za a iya ɗaukarsa azaman crystal ɗaya ko polycrystal ba, graphite yanzu gabaɗaya ana ɗaukarsa azaman kristal gauraye.
Lokacin aikawa: Yuli-31-2023